
(redirected from Isotopic mass)
The total number of neutrons and protons (symbol A), or mass number, of the nucleus gives approximately the mass measured on the so-called atomic-mass-unit (amu) scale. The numerical difference between the actual measured mass of an isotope and A is called either the mass excess or the mass defect (symbol Δ; see table). The atomic weights are available for elements 1 through 118 and isotopic compositions or abundances are given when appropriate. The atomic weights data were published by J. Meija et al in Atomic Weights of the Elements 2013, and the isotopic compositions data were published by M. Berglund and M.E. Wieser in Isotopic Compositions of the. Atomic Mass Isotopic Composition Standard Atomic Weight Notes: 1: H: 1: 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m: D: 2: 2.014 101 778 12(12) 0.000 115(70) T: 3: 3.016 049 2779(24). In the general chemistry series we learned about nuclide symbols, which all imply a specific atomic number and mass number. Associated with this were isotope. As mentioned in the previous section, atoms that have the same atomic number (number of protons), but different mass numbers (number of protons and neutrons) are called isotopes (nuclides). There are naturally occurring isotopes and isotopes that are artificially produced. Of all the elements on the periodic table, only 21 are pure elements.

Also found in: Dictionary, Thesaurus, Encyclopedia.
mass
[ mas]
mas] 
a·tom·ic mass
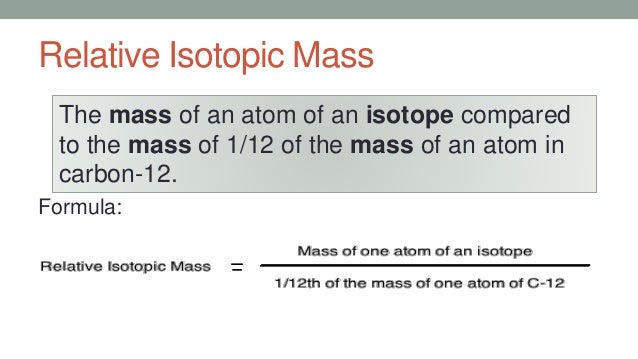
(ă-tomik mas)Isotopic Mass Formula
Want to thank TFD for its existence? Tell a friend about us, add a link to this page, or visit the webmaster's page for free fun content.
Link to this page:
Isotopic Mass Of Hydrogen
Website grabber mac. Isotopes are atoms of the same elements with same number of protons but different number of neutrons.
Many a times, students taking GCE A-Level H2 Chemistry are required to calculate the Relative Atomic Mass of an element with given information on Isotopic Abundance. The abundance of an isotope is the percentage of the isotope found in the naturally occurring element.

It might be given in Atomic Ratio or % Abundance.
Isotopic Mass Unit
Let’s check out an exam-based question that gives you the information in terms on Atomic Ratio.
Example:
Mountain lion on unsupported mac. The element rhenium consists of two isotopes 185Re and 187Re, in the atomic ratio of 2:3. Calculate the relative atomic mass of rhenium to three significant figures.
Suggested Answer:
Relative Atomic Mass, Ar = [(2 X 185) + ( 3 X 187)] / (2 + 3) = 186 (3 sig fig)
Isotopic Mass Questions
If the information given is in terms of % Abundance, the strategy to solve it is the same – just take the denominator to be 100 since % must add up to 100%.
The best way to learn is to take actions and work on it yourself. Try the question below and leave down your suggested answer.
Quick Check 1: Mac os yosemite google drive.
Chlorine has the following isotopes:
- 35Cl with relative isotopic mass of 34.97 and % abundance of 75.53
- 37Cl wirh relative isotopic mass of 36.97 and % abundance of 24.47
Calculate the relative atomic mass of chlorine.
PS: Try it out! Leave your answers in the comment section below.
Related Articles:
If you want to have an enjoyable time learning Chemistry and being motivated to excel in Chemistry, contact me today at 9828 7357
